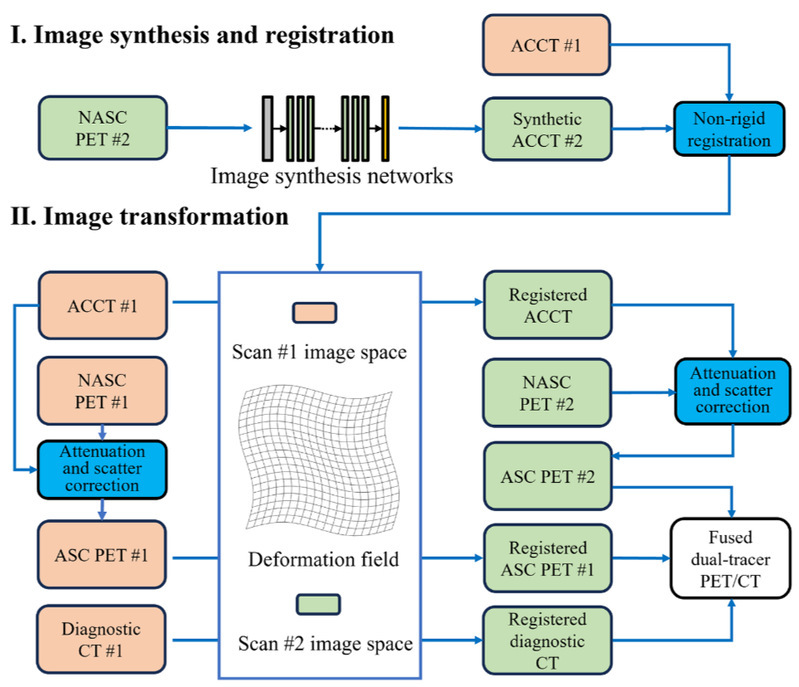
Eliminating the second CT scan of dual-tracer total-body PET/CT via deep learning-based image synthesis and registration
Yu Lin*, Kang Wang*, Zhe Zheng, Haojun Yu, Shuguang Chen, WenXin Tang, Yibo He, Huaping Gao, Runjun Yang, Yunzhe Xie, Junjie Yang, Xiaoguang Hou, Shuo Wang☨, Hongcheng Shi☨
European Journal of Nuclear Medicine and Molecular Imaging (IF=8.6)
Abstract
Purpose This study aims to develop and validate a deep learning framework designed to eliminate the second CT scan of dual-tracer total-body PET/CT imaging.
Methods We retrospectively included three cohorts of 247 patients who underwent dual-tracer total-body PET/CT imaging on two separate days (time interval:1–11 days). Out of these, 167 underwent [68Ga]Ga-DOTATATE/[18F]FDG, 50 underwent [68Ga]Ga-PSMA-11/[18F]FDG, and 30 underwent [68Ga]Ga-FAPI-04/[18F]FDG. A deep learning framework was developed that integrates a registration generative adversarial network (RegGAN) with non-rigid registration techniques. This approach allows for the transformation of attenuation-correction CT (ACCT) images from the first scan into pseudo-ACCT images for the second scan, which are then used for attenuation and scatter correction (ASC) of the second tracer PET images. Additionally, the derived registration transform facilitates dual-tracer image fusion and analysis. The deep learning-based ASC PET images were evaluated using quantitative metrics, including mean absolute error (MAE), peak signal-to-noise ratio (PSNR), and structural similarity index measure (SSIM) across the whole body and specific regions. Furthermore, the quantitative accuracy of PET images was assessed by calculating standardized uptake value (SUV) bias in normal organs and lesions.
Results The MAE for whole-body pseudo-ACCT images ranged from 97.64 to 112.59 HU across four tracers. The deep learning-based ASC PET images demonstrated high similarity to the ground-truth PET images. The MAE of SUV for whole-body PET images was 0.06 for [68Ga]Ga-DOTATATE, 0.08 for [68Ga]Ga-PSMA-11, 0.06 for [68Ga]Ga-FAPI-04, and 0.05 for [18F]FDG, respectively. Additionally, the median absolute percent deviation of SUV was less than 2.6% for all normal organs, while the mean absolute percent deviation of SUV was less than 3.6% for lesions across four tracers.
Conclusion The proposed deep learning framework, combining RegGAN and non-rigid registration, shows promise in reducing CT radiation dose for dual-tracer total-body PET/CT imaging, with successful validation across multiple tracers.